Services
GxP Consulting
i-Pharm GxP
Life Science Consulting Services to scope, staff, manage, and deliver GxP project solutions across North America, Europe, and the Asia-Pacific. With over 15 years of experience meeting business-critical needs for our partners around the world, our team will work with you to scope your requirements, identify key risks and opportunities, and agree on milestones for successful delivery.
We believe that every challenge is unique, which is why we adopt a tailor-made Scope of Work for each engagement; creating a roadmap for delivery that is purpose-built to your specific needs.
Previous examples include validating aseptic manufacturing equipment, processes, and systems, implementing a new gLIMS, responding to time-critical external audits, delivering successful biological license applications, designing new QC training programs and lab event workflows, and providing CQV oversight for the construction of a new state-of-the-art manufacturing facility.
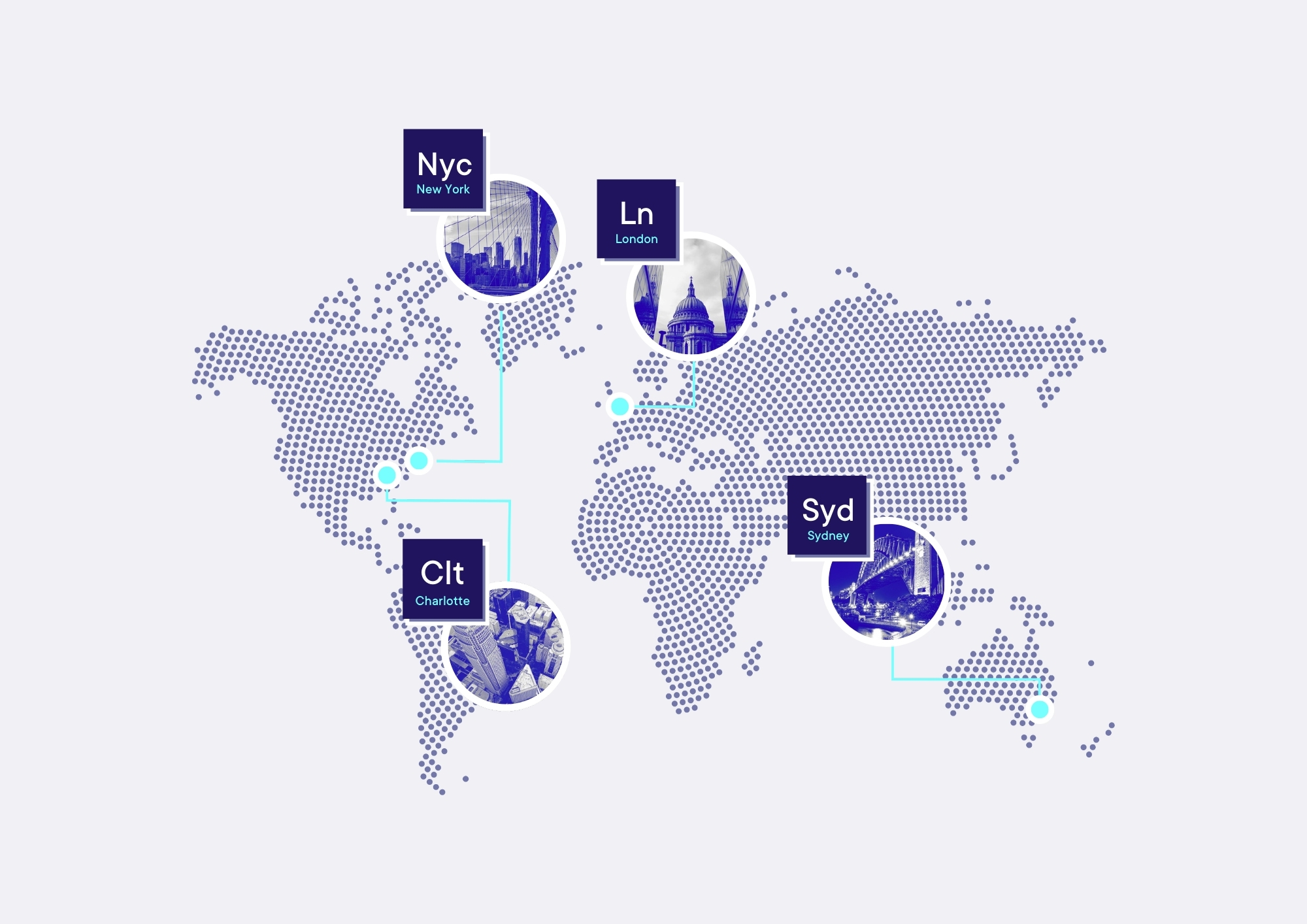
Our Markets
Our teams of SME Consultants work in partnership with our clients to enable the compliant, efficient supply of niche-regulated products to patients across three distinct areas within global life sciences.
Sterile / Aseptic Manufacturing
With a 15-year history of working directly with the world’s leading Sterile and Aseptic Manufacturers as a specialist, i-Pharm Consulting has a reputation for both trust and long-term value.
Biologics / Cell & Gene Therapy
Supporting our partners to develop and manufacture complex biologics represents a third of our global business and i-Pharm is proud to assist clients in spearheading the development of tomorrow’s treatments.
Medical Devices & Combination Products
We offer specialist, tailored support to Class I, II, and III Medical Devices and Combination Products, ensuring their safe and reliable delivery to patients.
What we do
"i-Pharm GxP ensures patient access to life-saving therapeutics through human-centric partnerships, projects, and solutions."
i-Pharm GxP offers the exclusive commitment of an expert consulting partner specializing in the tactical execution of specific project initiatives, as opposed to strategic consulting advice.
We give our clients the trusted technical bandwidth they need to address business-critical initiatives without disruption to BAU activities. The work we do with our partners is designed to:
- Improve efficiencies of product to market (through process, system, equipment engineering/AI/Automation services)
- Improve synergies with the ever-changing regulatory landscape for new, developing, or existing therapeutics (ensuring compliance with regulatory bodies such as the FDA, MHRA, EMA, PMDA, and CDSCO)
i-Pharm GxP is engaged to impact lasting change and we commit to providing your organization with the skills, knowledge, and personnel necessary to resolve your issues beyond the end of our specific engagement.
Intelligent Networks
We offer the perfect balance of a precise and agile workforce, designed to ensure a perfect fit and complete control for our partner's project needs.

Innovative Solutions
We strategically assess each and every engagement, each and every time in order to build a custom solution and deliver value for money.
Inclusive Relationships
We commit to solving our partner's problems in-house and work hard to impact lasting change through knowledge transfer, training, and development.
Our Technical Capabilities
We scope, staff, manage and deliver business-critical project initiatives for clients with capabilities in the following areas.

Quality Assurance
- Root Cause Investigation Analysis and Corrective & Preventative Action (CAPA) design and delivery
- Quality Operations Oversight & continuous manufacturing support
- Supper Quality Audit & Assessment
- QMS Assessment, Development & Optimisation
- Hypercare/Hypervigiliance services (CMO/CDMO oversight)
- Lean Six Sigma, DMAIC, fishbone, 5-whys methodologies, ISO 9001 & GEMBA/process walk-downs
Quality Control
- Microbiology & Immunology Support
- Lab Event Workflow Analysis
- Assay Development & Testing
- Analytical Instrument Audits
- Environmental Monitoring
Audit & Remediation Services
- Inspection Readiness
- Mock Audits/ Audit Preparation
- Pre-Approval Inspections (PAI)
- Remediation Services
- Audits of Critical Utilities, Laboratories, Manufacturing/Production facilities, and Warehousing/storage areas
- Gap Analysis and Priority/Impact Action Plans
- Working experience supporting Regulatory Bodies including FDA, MHRA, EMA, COFEPRIS, PMDA
Commissioning, Qualification and Validation Engineering
- Commissioning, Qualification, and Verification Services
- Computer Systems, Process and Equipment Validation
- Facilities Engineering
- Process Engineering
- IQ, OQ, PQ
- HVAC/facilities, smoke studies, air balancing, temperature mapping, environmental monitoring
Computer & Control Systems
End-user optimization/harmonization, training, development, and implementation services for:
- QMS (Trackiwse, eQMS, MasterControl etc)
- LIMS
- RIMS
- Labware
- MasterData
- KNEAT
- Netsuite
- Oracle ERP Cloud
- Johnson Controls, GE Digital, Siemens, Honeywell, Emerson Systems
AI / Machine Learning
- Automation System implementation and optimization services (Metasys, Rockwell, etc)
- Bioinformatics and pipeline buildouts through machine learning deployment and architecture building
- Data Cleansing
- Cloud Migration
- Data and infrastructure scaling and buildout

Technical Writing / Medical Writing
- Technical documentation authoring, review, editing, and approval – SOPs, Work Instructions, Batch Record Review, etc.
- Grant writing, scientific writing, editing/copy editing. Regulatory documentation, SOPs, CERs, CSRs, MDD/MDR, Submissions
- Writing protocols, clinical study reports, investigator brochures, and IND components
Regulatory Affairs
Working experience with Regulatory Bodies including FDA, MHRA, EMA, COFEPRIS, and PMDA supporting:
- Investigational New Drug (IND)
- New Drug Application (NDA)
- Abbreviated New Drug Application (ANDA)
- Orphan Drug Designation (ODD) and
Biologics License Applications (BLA)
Investigational Device Exemptions (IDE)
510 (K) submissions and
Pre-Market Approval (PMA)CMC strategy management and delivery
Clinical Operations
- Functional Service Provider (FSP)
- Clinical Trial Oversight & Management
- Clinical Operations
- Biostatistics
- Data Management
Scoping Your Project
Understanding the needs of our clients in detail is essential to our service offering. Every client, every assignment, and every location brings a new challenge to explore and ultimately overcome.
We believe that people make projects and the better we understand our client's objectives, challenges and current team structures the better we can shape our business to deliver success to each initiative.
We take the time to understand every detail: not just the technical, but the cultural, logistical, and ultimately human nature of each challenge.
Every scope is treated with confidence and protected by NDAs that ensure we can speak with the openness and transparency necessary to design the right solution.

Scope a project with i-Pharm GxP
SubmitMatching Timelines
We will move as quickly as your timelines allow and can deploy consultants within 2 weeks of a detailed brief.
Our clients enjoy the benefit of an agile workforce that can ramp up and down the critical path of each project.
Highest Quality Personnel
Our rigorous vetting process allows us to present only the best-suited professionals for each project. We do not operate a bench model and employ technical testing, 360 reference checks, and face-to-face meetings at our global offices to ensure our partners benefit from the best talent in the market.
Retention
We have a 96% retention rate for consultants on assignment because we believe that People make Projects.
Selection and retention of the right people for the right reasons is key to project continuity and success for our partners.
Governance
Management and delivery of milestones and objectives are crucial for the success of each project. Our partners control the level of governance required for each scope, whether we are deploying a team of 30 or a team of 3 and we commit to regular stakeholder reviews and project plans.
Transparent Pricing
We operate with fairness, transparency, and balance when it comes to pricing each of our engagements. Our business philosophy is underpinned by a desire for repeat, referral, and relationship-based business which we gain only through delivering long-term value for our partners.
Knowledge Transfer
All of our work product is ultimately owned by our partners. Wherever possible we focus on knowledge transfer, training, and development of our partners' FTE workforce to ensure we deliver lasting change in the industry and adhere to our human-centric mission statement.
Say hello to your GxP team
Listen to the i-Pharm GxPodcast
The GxPodcast by i-Pharm GxP is a podcast that shares conversations of thought leadership and perspectives on GxP solutions in the life sciences industry. Click below to listen in!








