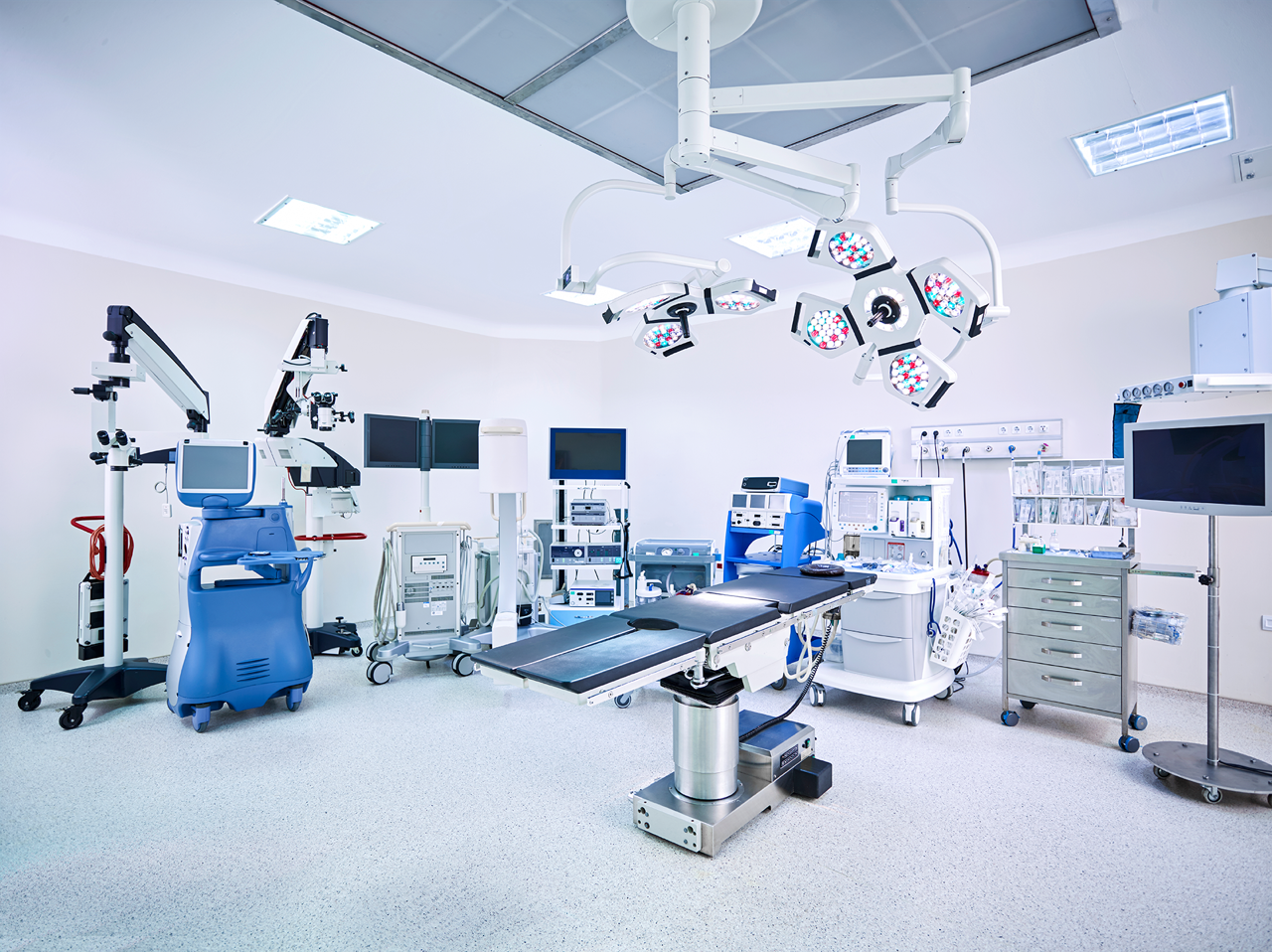
GxP Consulting
Medical Devices / Combination Products
Tailored Support
We offer specialist, tailored support to Class I, II, and III Medical Devices and Combination Products, ensuring their safe and reliable delivery to patients.
With a global lens, our consultants at i-Pharm GxP expertly navigate the intricate regulatory requirements of multiple regions. In the U.S., our depth of expertise encompasses the FDA's QSR 21 CFR Part 820, MDR, and cGMP practices. Additionally, in Europe, we are well-versed in the EU MDR 2017/745 and EU IVDR 2017/746. This international perspective guarantees that our partners' products align seamlessly with standards, irrespective of the targeted market.
Beyond the initial product launch, our commitment extends to overseeing post-market adjustments such as product updates or modifications, ensuring continuous regulatory compliance. Our unwavering dedication to quality, safety, and adaptation is reflected in the successful stories we've co-authored with our partners in the ever-evolving medical device arena.
Our Technical Capabilities
We scope, staff, manage and deliver business-critical project initiatives for clients with capabilities in the following areas.

Quality Assurance
- Root Cause Investigation Analysis and Corrective & Preventative Action (CAPA) design and delivery
- Quality Operations Oversight & continuous manufacturing support
- Supper Quality Audit & Assessment
- QMS Assessment, Development & Optimisation
- Hypercare/Hypervigiliance services (CMO/CDMO oversight)
- Lean Six Sigma, DMAIC, fishbone, 5-whys methodologies, ISO 9001 & GEMBA/process walk-downs
Quality Control
- Microbiology & Immunology Support
- Lab Event Workflow Analysis
- Assay Development & Testing
- Analytical Instrument Audits
- Environmental Monitoring
Audit & Remediation Services
- Inspection Readiness
- Mock Audits/ Audit Preparation
- Pre-Approval Inspections (PAI)
- Remediation Services
- Audits of Critical Utilities, Laboratories, Manufacturing/Production facilities, and Warehousing/storage areas
- Gap Analysis and Priority/Impact Action Plans
- Working experience supporting Regulatory Bodies including FDA, MHRA, EMA, COFEPRIS, PMDA
Commissioning, Qualification and Validation Engineering
- Commissioning, Qualification, and Verification Services
- Computer Systems, Process and Equipment Validation
- Facilities Engineering
- Process Engineering
- IQ, OQ, PQ
- HVAC/facilities, smoke studies, air balancing, temperature mapping, environmental monitoring
Computer & Control Systems
End-user optimization/harmonization, training, development, and implementation services for:
- QMS (Trackiwse, eQMS, MasterControl etc)
- LIMS
- RIMS
- Labware
- MasterData
- KNEAT
- Netsuite
- Oracle ERP Cloud
- Johnson Controls, GE Digital, Siemens, Honeywell, Emerson Systems
AI / Machine Learning
- Automation System implementation and optimization services (Metasys, Rockwell, etc)
- Bioinformatics and pipeline buildouts through machine learning deployment and architecture building
- Data Cleansing
- Cloud Migration
- Data and infrastructure scaling and buildout

Technical Writing / Medical Writing
- Technical documentation authoring, review, editing, and approval – SOPs, Work Instructions, Batch Record Review, etc.
- Grant writing, scientific writing, editing/copy editing. Regulatory documentation, SOPs, CERs, CSRs, MDD/MDR, Submissions
- Writing protocols, clinical study reports, investigator brochures, and IND components
Regulatory Affairs
Working experience with Regulatory Bodies including FDA, MHRA, EMA, COFEPRIS, and PMDA supporting:
- Investigational New Drug (IND)
- New Drug Application (NDA)
- Abbreviated New Drug Application (ANDA)
- Orphan Drug Designation (ODD) and
Biologics License Applications (BLA)
Investigational Device Exemptions (IDE)
510 (K) submissions and
Pre-Market Approval (PMA)CMC strategy management and delivery
Clinical Operations
- Functional Service Provider (FSP)
- Clinical Trial Oversight & Management
- Clinical Operations
- Biostatistics
- Data Management
Trusted Partner
As a trusted partner in the medical device field, we recognise the importance of a patient-first approach and a commitment to fostering holistic cultural change.
Our focus extends beyond our project teams and the SOPs and engineering upgrades we validate, as we also prioritise knowledge transfer and the development of long-lasting, day-to-day practices that ensure patient safety.
Comprehensive Solutions
At i-Pharm GxP, we work closely with our clients to cultivate an understanding of their unique needs and challenges, enabling us to deliver comprehensive solutions that have a lasting impact on patient safety and overall device performance. By emphasising collaboration and communication, we are able to help our partners navigate the complex landscape of medical devices and combination products with confidence.
Choose i-Pharm GxP as your specialist Medical Device and Combination Products partner and let us guide you through the intricate process of developing, manufacturing, and ensuring regulatory compliance for your innovative solutions.